MakeTheBrainHappy Is PCl3 Polar or Nonpolar?
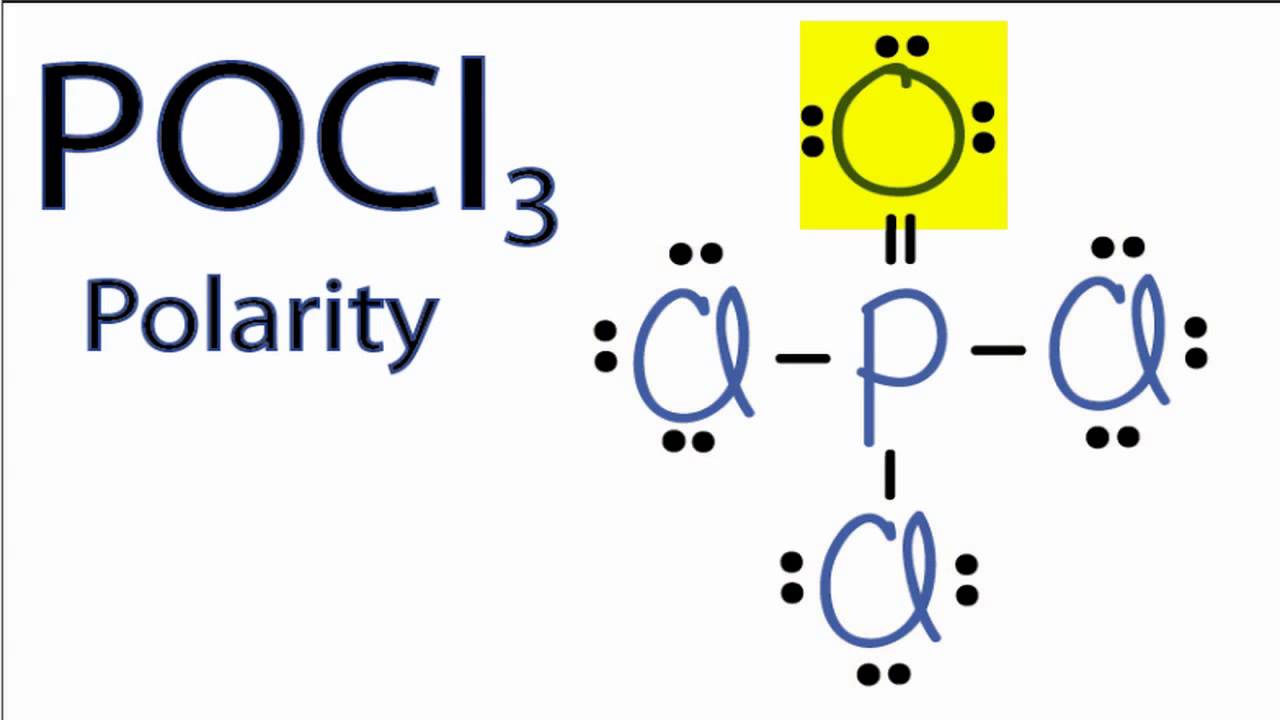
POCl3 is a POLAR molecule because the P=O bond and P-Cl bonds present in the molecule are polar and it has asymmetric geometry which causes the partial positive (ẟ+) and partial negative (ẟ-) charge to appear on the molecule. These ẟ+ and ẟ- charges are responsible to make the entire POCl3 molecule polar.

Is PCL3 Polar or Nonpolar? (Phosphorus Trichloride) YouTube
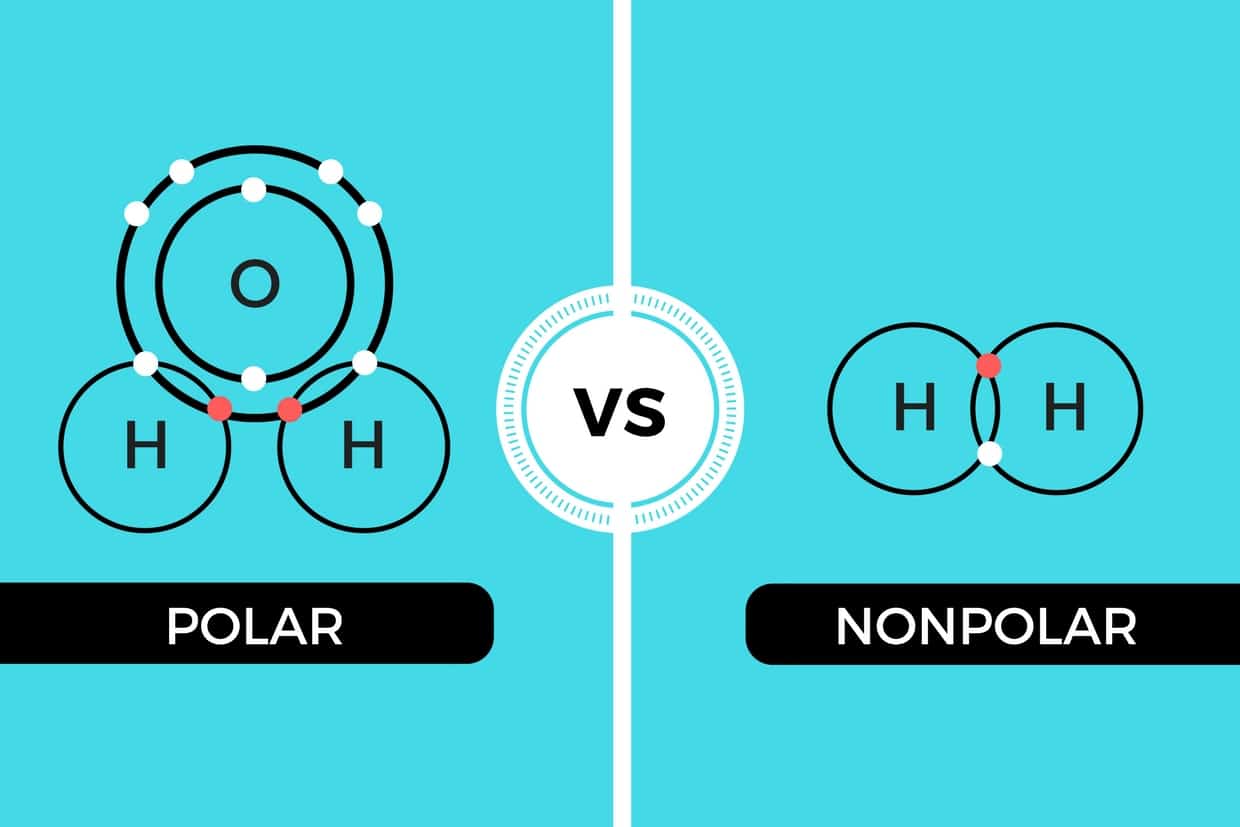
The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.

Polar and Nonpolar Molecules
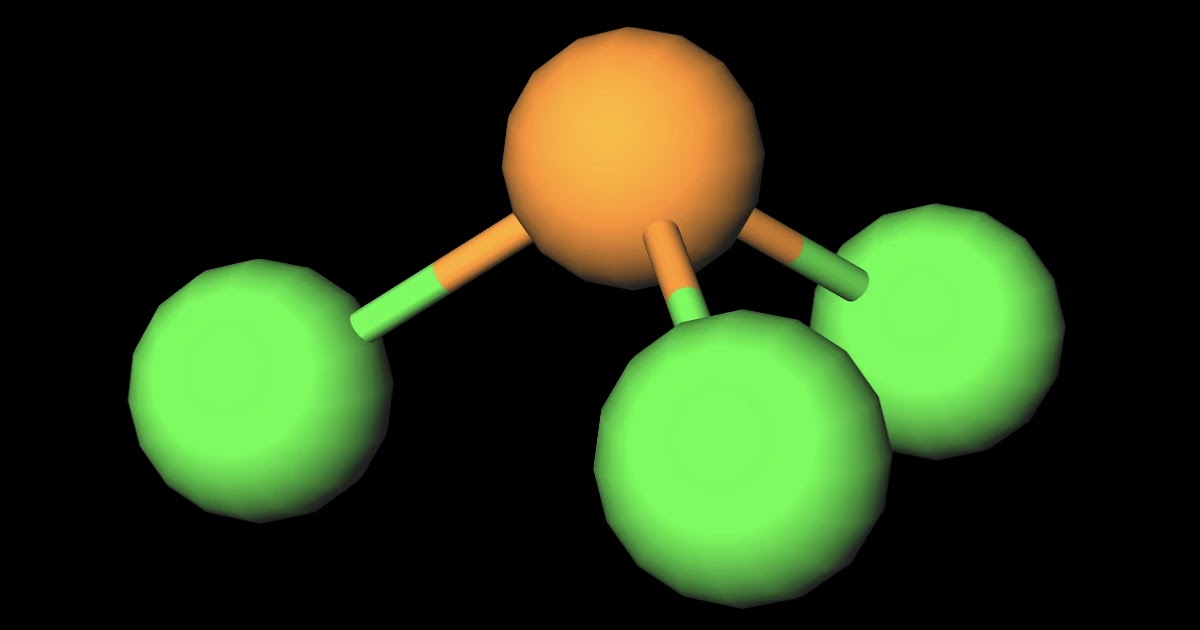
The PCl3 is a polar molecule, and the polarity is due to its tetrahedral geometry with a lone pair of electrons on the phosphorus atom. The electronegativity difference between chlorine and phosphorus creates two opposing dipoles with a more negative charge on chlorine and a positive charge on phosphorus results in polar bond formation.
Is POCl3 Polar or Nonpolar? YouTube
Despite the polar bonds, PCl3 is a nonpolar molecule due to its symmetrical arrangement. PCl3 Lewis Structure Bond Angle. The bond angle in PCl3, or phosphorus trichloride, is a crucial aspect of its molecular geometry. Understanding the bond angle helps us comprehend the overall shape and properties of the molecule.

Is \ce{PCl3} polar or nonpolar? Quizlet
Is PCl3 polar or nonpolar? Don't worry, the answer is simple! PCl3 is a polar molecule because of its geometry and difference in electronegativity between the 2 atoms. Again another question like, whether PCl3 is ionic or covalent, can pop up in your mind.
Pcl3 Polar Or Nonpolar Asking List
In a polar covalent bond, sometimes simply called a polar bond, the distribution of shared electrons within the molecule is no longer symmetrical (see figure below). Figure 5.3.4 5.3. 4: In the polar covalent bond of HF HF, the electron density is unevenly distributed. There is a higher density (red) near the fluorine atom, and a lower density.
Polar and Nonpolar Covalent Bonds Characteristics & Differences
PCl3 is a polar molecule because of its tetrahedral geometrical shape having a lone pair on Phosphorus atom and the difference between the electronegativity of Chlorine (3.16) and Phosphorus (2.19) atoms resulting in unequal sharing of electrons and develop positive and negative poles across the molecule making it a polar molecule.

Is PCl3 Ionic or Covalent? Techiescientist
Phosphoryl Chloride or Phosphorus Oxychloride is a polar molecule due to the uneven distribution of valence electrons in the molecule and a net dipole moment in it. Due to the asymmetry seen in the molecule's shape, the dipole moments are not nullified, making this molecule a polar molecule.
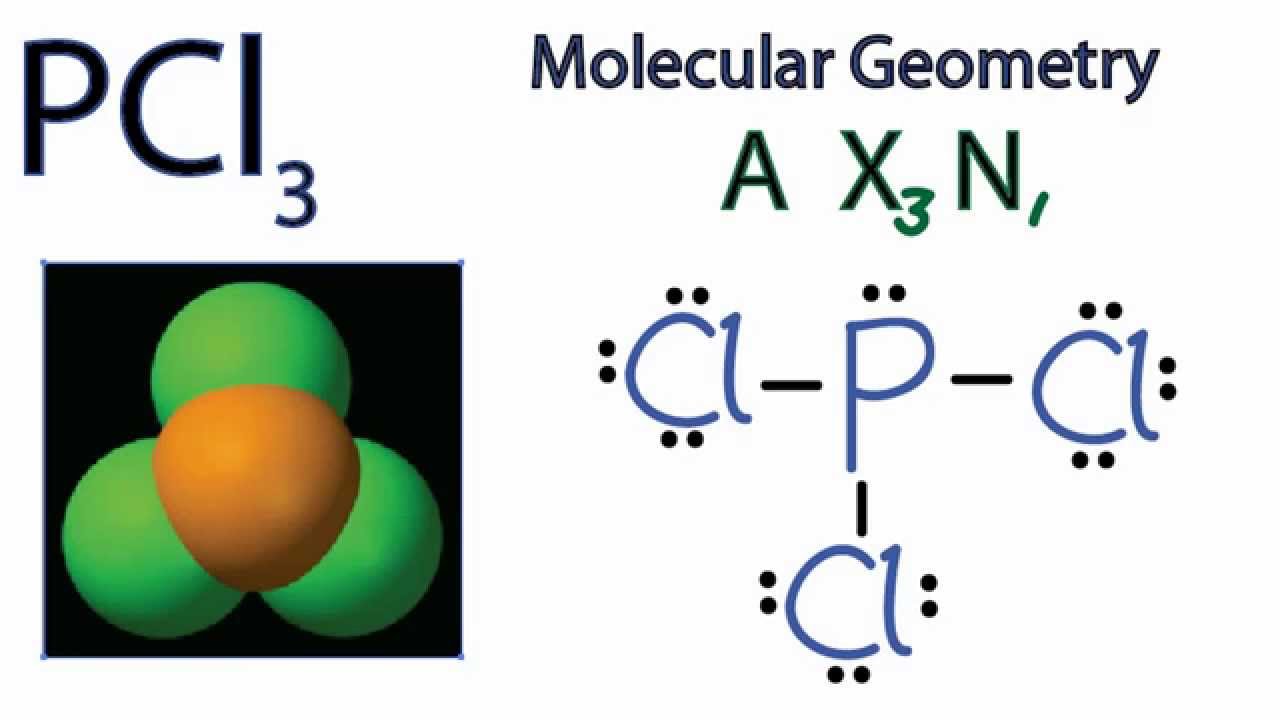
PCl3 Molecular Geometry / Shape and Bond Angles YouTube
Conclusion FAQ on "Is PCl3 polar or nonpolar?" Is PCl3 a polar compound? What type of bond is PCl3? Is PCl3 a dipole?

PCl3 Lewis Structure and Molecular Geometry YouTube
Hey Guys!In this video, we are going to determine the polarity of Phosphorus Trichloride having a chemical formula of PCL3.To know the polarity of this molec.
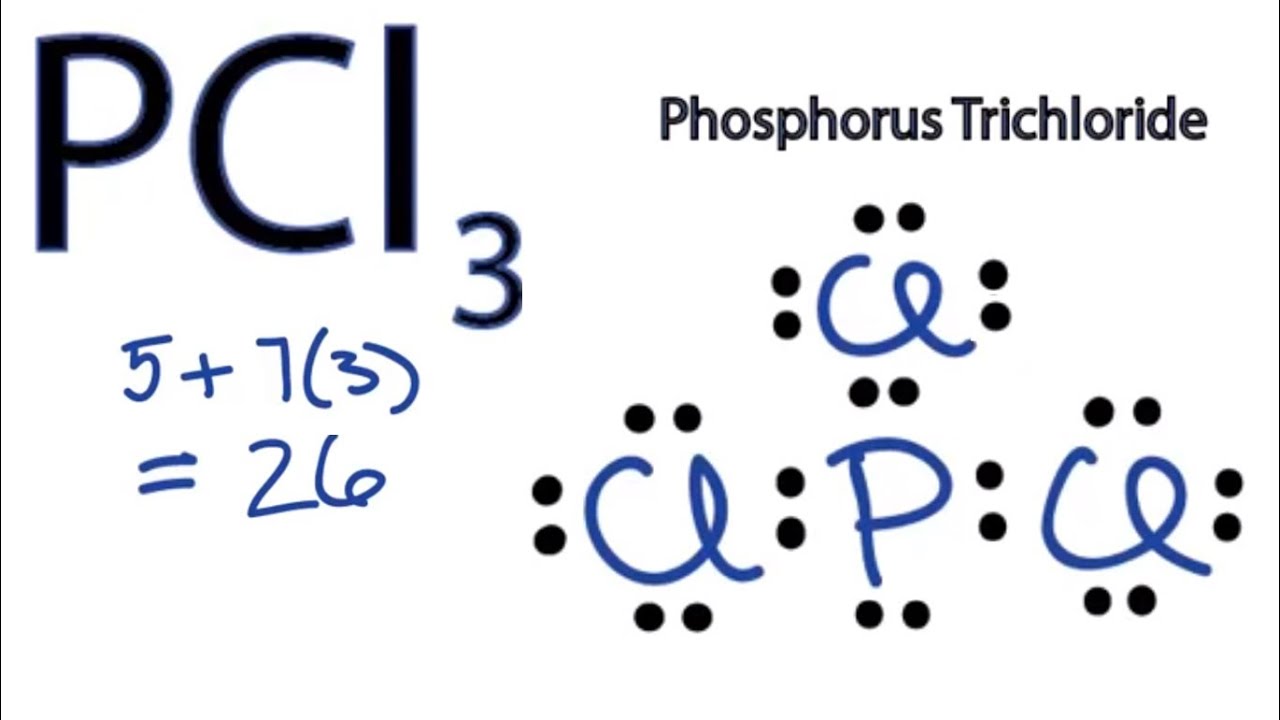
What Is Pcl3 Lewis Structure?
Formula: The chemical formula of phosphorous trichloride is (PCL3). Common Name: The common name of PCL3 is Trichlorophosphane, Phosphorous chloride, and Phosphorus (III) chloride. Table of Contents Is PCL3 Polar Or Nonpolar Summary PCL3 Molecular Geometry Summary PCL3 Lewis Structure Hybridization Of PCL3 Summary Bond Angle Of PCL3 Summary

Is PCl3 Polar or NonPolar? (Phosphorous trichloride) Yes Dirt
Is PCl3 polar or nonpolar? Question: Is PCl 3 polar or nonpolar? VSEPR: VSEPR is the acronym representing ''valence shell electron pair repulsion.'' It's a theory that utilizes the electron.

Is PCl3 (Phosphorus trichloride) Ionic or Covalent/Molecular? YouTube
Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas. It is a toxic compound but is used in several industries. Phosphorus Trichloride is widely used in manufacturing Phosphites and other organophosphorus compounds.

Pcl3 Polar Or Nonpolar Asking List
Phosphorous trichloride molecules are polar because each atom contributes unevenly to the molecule's electron density, making it unequally charged along its three axes.
How To Know If A Molecule Is Polar Or Nonpolar Khan Academy
PCl 3 is a polar molecule because the P-Cl bond is polar, and the three bonds are not equivalent to the lone pair which causes an asymmetrical distribution of bonding electrons in the molecule. This results in a permanent dipole directed towards the P-Cl bonds as drawn: Check this 99-question multiple-choice quiz on Geometry and Hybridization: Free
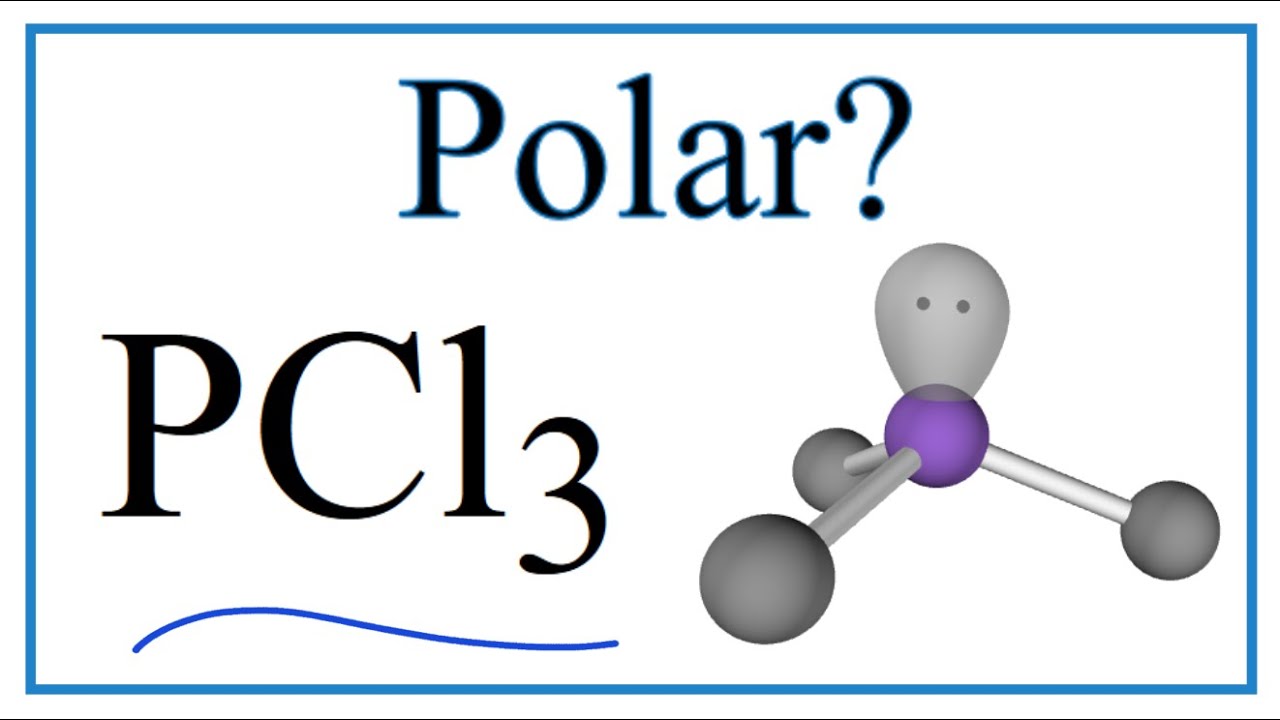
Is PCl3 (Phosphorous trichloride) Polar or NonPolar YouTube
In order to know whether PCl3 is a polar covalent molecule or nonpolar covalent molecule, we have to check the electronegativity difference of the combining atoms. If the electronegativity difference ( ΔEN ) is less than 0.4 , then the bond is nonpolar covalent bond.